A groundbreaking discovery has emerged in the fight against Alzheimer’s, as scientists unveil a new drug that has shown promise in slowing the progression of the disease. Dubbed Donanemab, this remarkable medication has been hailed as a potential “turning point” by experts in the field.
The key finding from a trial of Donanemab is that it can slow the “clinical decline” in Alzheimer’s patients by an impressive 35%. This means that those affected by the disease can maintain their ability to carry out daily tasks, such as shopping, housekeeping, managing finances, and taking medication.

Alzheimer’s Research UK has expressed their optimism, stating that we may be entering a new era where Alzheimer’s becomes a treatable condition. Similarly, the Alzheimer’s Society envisions a future where Alzheimer’s disease is akin to long-term conditions like asthma or diabetes, indicating that this breakthrough could mark the beginning of the end for this devastating illness.
TRAILBLAZER ALZ-2 Trial Demonstrates Efficacy of Donanemab
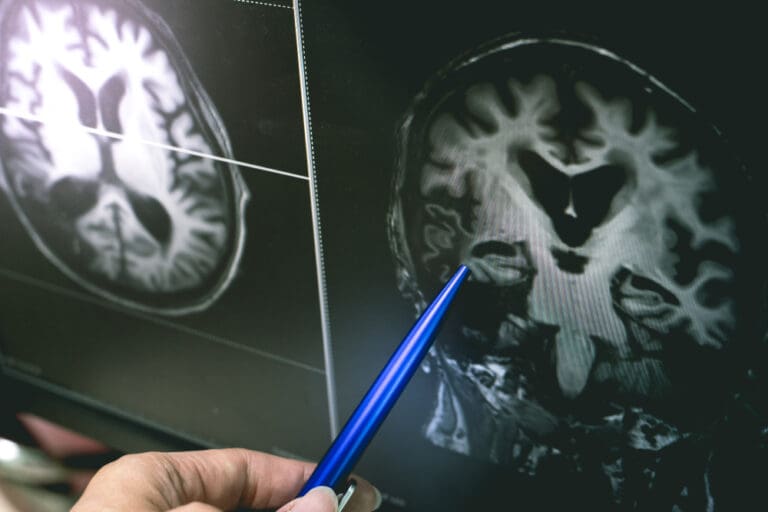
The mechanism of Donanemab involves removing harmful plaques of a protein known as amyloid that accumulate in the brains of Alzheimer’s patients. The drug, manufactured by Eli Lilly, was tested in a trial called TRAILBLAZER ALZ-2, involving nearly 1,800 individuals with early-stage Alzheimer’s. Half of the participants received monthly infusions of Donanemab, while the other half received a placebo over an 18-month period.

The trial results, published in the Journal of the American Medical Association and presented at the Alzheimer’s Association International Conference, demonstrated an impressive 35.1% slowing of clinical decline in individuals with early-stage Alzheimer’s and low or medium levels of tau protein when treated with Donanemab for 76 weeks. When considering all participants with varying levels of tau protein, the disease progression was slowed by 22.3%.
Notable Side Effects Observed, Including Brain Swelling
While the results are highly promising, there were some notable side effects observed in a small number of participants, including brain swelling. Additionally, the study identified three deaths in the Donanemab group and one in the placebo group that were considered “treatment related.”
Eli Lilly noted that some patients on Donanemab could complete their treatment course in just six months once their amyloid plaque cleared. The drug showed an average reduction of 84% in amyloid plaque at 18 months, while the placebo group saw only a 1% decrease.
Another Promising Drug in the Fight Against Alzheimer’s

It’s worth noting that a separate drug, Lecanemab, has also shown encouraging results, slowing Alzheimer’s symptoms by 27% in patients in the early stages of the disease. The US has already approved Lecanemab for use.
Dr. Mark Mintun, group vice president of neuroscience research and development at Eli Lilly, commented on the significance of these findings, emphasizing the importance of diagnosing and treating the disease at an early stage to achieve the best outcomes.
Experts Emphasize Importance of Early Diagnosis and Access to Treatment
Dr. Richard Oakley, associate director of research at Alzheimer’s Society, echoed the sentiment, calling this a turning point in the fight against Alzheimer’s. He emphasized the potential for Alzheimer’s to be considered a long-term condition with effective symptom management through treatments like Donanemab and Lecanemab. However, he highlighted the critical role of early and accurate diagnosis in ensuring broad access to these treatments, urging the need for widespread availability of early diagnostic methods through the NHS.